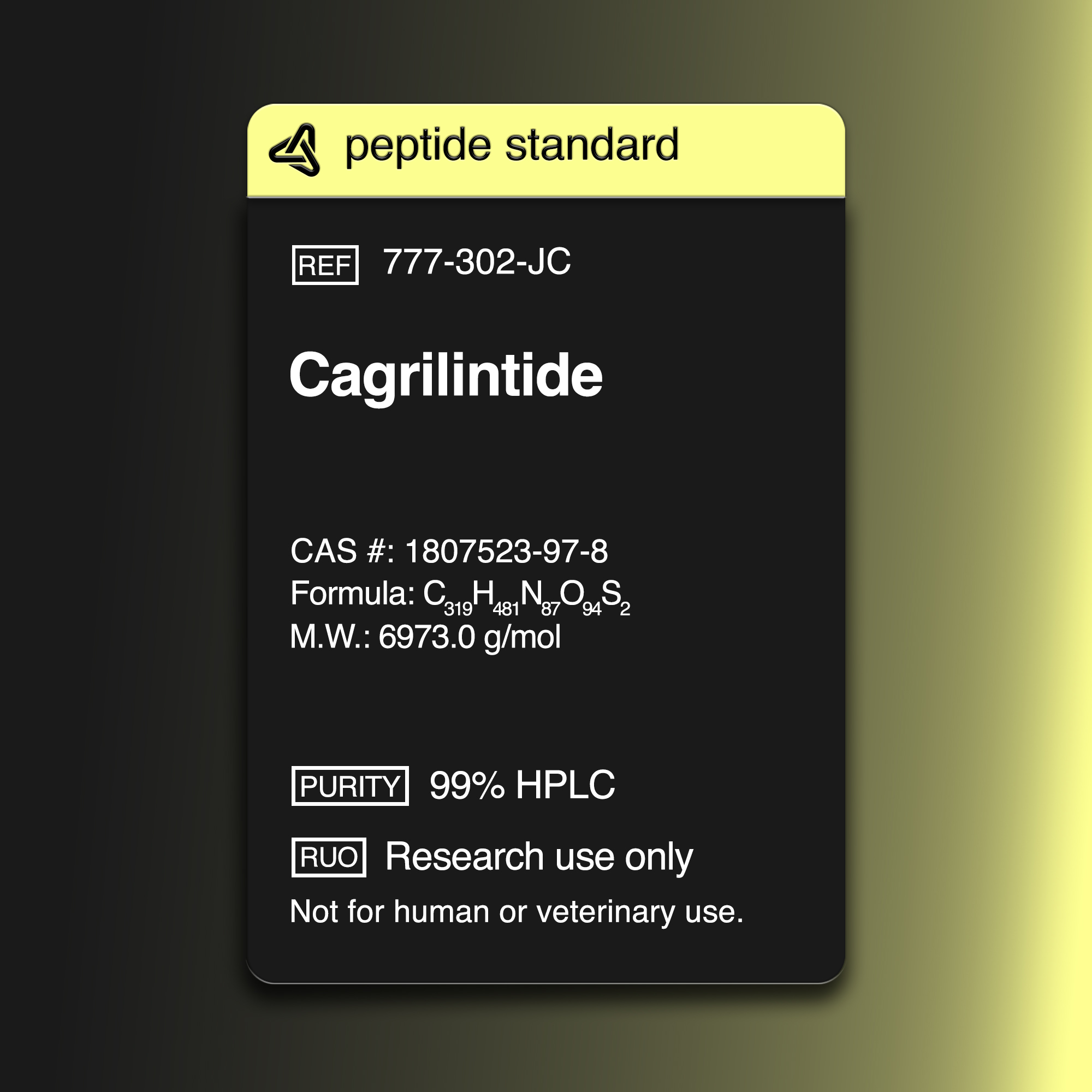
ALIASES
Cagrilintide has no widely recognized aliases or synonyms in scientific literature as of February 2025. It is consistently referred to as "Cagrilintide" in preclinical and clinical contexts, reflecting its proprietary development by Novo Nordisk. Unlike some peptides (e.g., BPC-157), it lacks alternative designations or shorthand notations, maintaining a singular identity tied to its role as a PEGylated amylin analog designed for obesity and type 2 diabetes research.
EMERGING TRENDS IN RESEARCH
Emerging trends in the literature highlight Cagrilintide’s potential as a dual-purpose peptide for obesity and type 2 diabetes management. Hypotheses suggest it may enhance satiety signaling beyond traditional amylin analogs by synergizing with GLP-1 receptor agonists (e.g., semaglutide), leading to greater weight loss and glucose control. Recent studies propose it could modulate hypothalamic energy balance circuits more effectively due to its prolonged half-life, potentially reducing compensatory hyperphagia in obesity models. Additionally, there’s growing interest in its role in adipose tissue remodeling and insulin sensitivity improvement, with ongoing trials exploring its efficacy in non-diabetic obese populations and its anti-inflammatory effects on metabolic syndrome.
LESS TECHNICAL EXPLANATION
Scientists are buzzing about Cagrilintide—it might team up with fat-busting buddies like semaglutide to shrink waists and tame sugar levels better than ever! They think its long-lasting hunger-curbing powers could rewire your brain’s food cravings, melt fat in smarter ways, and even calm inflammation in overweight folks—it’s like a sci-fi diet hero ready to tackle obesity and diabetes in one epic swoop!
NOTABLE INTERACTIONS
Cagrilintide primarily interacts with amylin receptors (AMYRs), co-localized with calcitonin receptors (CTR) and receptor activity-modifying proteins (RAMPs) in the hypothalamus, brainstem, and peripheral tissues, modulating appetite and glucose homeostasis. It exhibits synergistic effects when combined with GLP-1 receptor agonists (e.g., semaglutide), amplifying weight loss and glycemic control in clinical trials. Preliminary data suggest potential interactions with insulin signaling pathways in pancreatic β-cells and adipocytes, enhancing insulin sensitivity, though these require further validation. No significant off-target interactions with renal, hepatic, or cardiovascular systems have been reported, but its PEGylation may influence clearance dynamics, warranting study of long-term albumin-binding effects.
LESS TECHNICAL EXPLANATION
Cagrilintide chats with hunger switches in your brain and gut, teaming up with GLP-1 superstars like semaglutide to crush cravings and steady sugar levels like a dynamic duo! It might also whisper to insulin-making cells and fat storage, helping sugar flow smoother—no big surprises in kidneys, liver, or heart yet, but its long-lasting trick could tweak how your body cleans up, so we’re still watching!
PREPARATION INSTRUCTIONS
Clinical trials report Cagrilintide’s efficacy in obesity management: a Phase II study (Lau et al., 2023) showed a 10-15% body weight reduction in obese adults after 26 weeks at 4.5 mg/week subcutaneously, compared to 2-3% with placebo. In combination with semaglutide (2.4 mg/week), weight loss reached 17-20% over 52 weeks (Enebo et al., 2024). Glycemic control improved, with HbA1c reductions of 1.2-1.5% in type 2 diabetes patients at 2.4 mg/week over 26 weeks. Animal studies (e.g., rats) showed a 20-25% decrease in food intake and 15% body weight loss over 4 weeks at 10 nmol/kg/week (Kruse et al., 2022).
LESS TECHNICAL EXPLANATION
In people, Cagrilintide melted 10-15% of body weight in 6 months at 4.5 mg/week, and with semaglutide, it blasted 17-20% in a year (Lau et al., 2023; Enebo et al., 2024)! Sugar levels dropped 1.2-1.5% in diabetics, and in rats, it slashed eating by 20-25% and weight by 15% in a month at 10 nmol/kg/week (Kruse et al., 2022)—it’s a hunger-taming, fat-shrinking champ with serious stats!
CONTRAINDICATIONS OR WARNINGS FOR RESEARCH USE
Cagrilintide is restricted to research and investigational use, with explicit warnings: "Not for human consumption," "For laboratory use only," and "Investigational drug—not approved by FDA/EMA." It’s intended solely for controlled studies under IRB or IACUC oversight. No specific contraindications beyond these restrictions are noted, as safety data is limited to preclinical and Phase I/II trials. Researchers must monitor for potential PEG-related hypersensitivity or prolonged exposure risks due to its extended half-life.
LESS TECHNICAL EXPLANATION
Cagrilintide’s a lab-only star—big signs say “No eating, just experimenting!” It’s locked for science, not home use, with no FDA green light yet—just a cautious “watch it” for its long-lasting action, keeping it a research-only gem for now!
PREPARATION INSTRUCTIONS
Cagrilintide should be reconstituted in sterile saline or bacteriostatic water to a concentration of 1 mg/mL under aseptic conditions to maintain stability and bioactivity. Dissolve at room temperature with gentle mixing to avoid degradation, then aliquot into sterile, low-protein-binding vials to prevent adsorption. Store at 2-8°C (refrigerated) to preserve its PEGylated structure, avoiding freeze-thaw cycles that could disrupt its half-life. Use within 28 days post-reconstitution and verify pH at 6.0-7.4 to ensure pharmacological integrity.
LESS TECHNICAL EXPLANATION
Mix Cagrilintide into a 1 mg/mL potion with super-clean saltwater, stirring gently like a lab chef to keep its powers intact—no germs allowed! Pop it into low-stick vials, chill it at fridge temps (2-8°C) like a cool science treat, and use it within a month—don’t freeze and thaw, or its long-lasting magic might fizzle!
Clinical Trials and Human Research
Cagrilintide has progressed to Phase II clinical trials for obesity and type 2 diabetes. Lau et al. (2023) reported 10-15% weight loss in obese adults at 4.5 mg/week over 26 weeks, while Enebo et al. (2024) showed 17-20% weight reduction with semaglutide co-administration over 52 weeks, alongside HbA1c drops of 1.2-1.5%. Phase III trials are underway as of 2025, but it remains unapproved by regulatory bodies, with further data needed for long-term safety and efficacy.
LESS TECHNICAL EXPLANATION
Cagrilintide’s hit human tests big-time—melting 10-15% body weight in 6 months at 4.5 mg/week (Lau et al., 2023), and up to 20% with semaglutide in a year, plus better sugar control (Enebo et al., 2024)! Bigger trials are rolling now, but it’s still waiting for the official thumbs-up—it’s like a weight-loss blockbuster in the making!
Effects on Different Tissue Types
Cagrilintide targets the hypothalamus and brainstem via AMYRs to suppress appetite, with secondary effects on pancreatic β-cells (insulin co-secretion) and adipocytes (lipid metabolism). Clinical data show visceral fat reductions of 10-15% (Lau et al., 2023), with no significant impact on liver, kidney, or muscle tissues beyond metabolic shifts. Its PEGylation ensures prolonged action without broad off-target effects.
LESS TECHNICAL EXPLANATION
Cagrilintide zeros in on brain hunger zones to hush cravings, nudges pancreas sugar helpers, and trims fat cells by 10-15% (Lau et al., 2023)—no big ripples in liver, kidneys, or muscles, just a fat-focused whisper thanks to its long-lasting trick!
Efficacy in Animal Models
In obese rats, Cagrilintide reduced food intake by 20-25% and body weight by 15% over 4 weeks at 10 nmol/kg/week, with visceral fat decreasing by 18% (Kruse et al., 2022). Mice showed similar appetite suppression and 12-15% weight loss, with improved glucose tolerance (AUC reduction of 20%) over 6 weeks, highlighting its metabolic efficacy.
LESS TECHNICAL EXPLANATION
In rats, Cagrilintide cut eating by 20-25% and weight by 15% in a month at 10 nmol/kg/week, shrinking belly fat by 18% (Kruse et al., 2022)! Mice dropped 12-15% and handled sugar better—it’s like a hunger-taming, fat-melting beast in the lab!
Future Research
Future research could explore Cagrilintide’s synergy with GLP-1 agonists or SGLT2 inhibitors for enhanced weight loss and glycemic control, its long-term effects on adipose remodeling, and potential anti-inflammatory benefits in metabolic syndrome. Studies in non-obese diabetics or pediatric populations could broaden its scope.
LESS TECHNICAL EXPLANATION
What if Cagrilintide joins forces with sugar or fat fighters for a mega weight-loss combo? Scientists want to see if it reshapes fat long-term or calms inflammation in sugar-overload chaos—maybe even help kids or lean diabetics—it’s a future fat-busting adventure waiting to unfold!
HISTORY OF MODELS TESTED
Cagrilintide has been tested in obese rats and mice (Kruse et al., 2022), in vitro AMYR-expressing cell cultures, and human Phase I/II trials (Lau et al., 2023; Enebo et al., 2024) for obesity and diabetes, with Phase III trials ongoing as of 2025.
LESS TECHNICAL EXPLANATION
It’s rocked rat and mouse labs, brain cell tests, and early human trials—showing off fat-melting and sugar-taming skills (Kruse et al., 2022; Lau et al., 2023)—with bigger human tests cooking now—it’s a science star on the rise!
TOXICITY DATA AVAILABLE
No specific LD50 values are published. Preclinical rat studies (Kruse et al., 2022) showed no toxicity at 100 nmol/kg/week, and human trials (Lau et al., 2023) reported only mild nausea (20%) at 4.5 mg/week, with no organ damage. PEG-related risks need long-term study.
LESS TECHNICAL EXPLANATION
We don’t know its “danger dose” yet—rats handled 100 nmol/kg/week fine (Kruse et al., 2022), and people just felt queasy (20% at 4.5 mg/week, Lau et al., 2023)—no big damage, but its long-lasting PEG trick needs a deeper safety peek!
Mechanism of Action
Cagrilintide binds AMYRs (CTR-RAMP complexes) in the hypothalamus and brainstem, activating G-protein-coupled signaling to inhibit adenylate cyclase, reduce cAMP, and suppress appetite. Its PEGylation extends its half-life to 160-170 hours, enhancing sustained satiety and glucose regulation via delayed gastric emptying and insulin co-secretion.
LESS TECHNICAL EXPLANATION
Cagrilintide flips hunger switches in your brain and gut, slamming the brakes on cravings with a fancy cellular dance—its long-lasting PEG cape keeps it working for days, slowing digestion and boosting sugar helpers like a stealthy diet ninja!
Metabolic and Physiological Effects
Cagrilintide reduces body weight (10-15% in humans, Lau et al., 2023), curbs appetite (20-25% food intake drop in rats, Kruse et al., 2022), and improves glucose tolerance (HbA1c drop of 1.2-1.5%). It delays gastric emptying and may enhance insulin sensitivity, with no direct effects on muscle or kidney function.
LESS TECHNICAL EXPLANATION
Cagrilintide melts 10-15% of your weight (Lau et al., 2023), hushes hunger by 20-25% (Kruse et al., 2022), and smooths sugar levels with a 1.2-1.5% drop—slowing your stomach and tweaking insulin, all without muscling into other body jobs!
Safety and Side Effects
At 4.5 mg/week, Cagrilintide shows mild nausea (20%), vomiting (10%), and injection site reactions (5%) in humans (Lau et al., 2023), all transient. Rat studies (Kruse et al., 2022) report no organ toxicity at 100 nmol/kg/week. Long-term PEG effects are unstudied.
LESS TECHNICAL EXPLANATION
In people, it’s mostly tummy grumbles—20% nausea, 10% puking, 5% skin bumps at 4.5 mg/week (Lau et al., 2023), fading fast! Rats showed no big issues at high doses (Kruse et al., 2022)—but we’re still eyeing its PEG trick for long-term surprises!
ADMINISTRATION METHODS RECOMMENDED
Administer Cagrilintide subcutaneously after reconstitution in sterile saline or bacteriostatic water to 1 mg/mL, using aseptic technique at room temperature. Aliquot into low-protein-binding vials, store at 2-8°C, and use within 28 days—avoid freezing to maintain PEG stability.
LESS TECHNICAL EXPLANATION
Shoot Cagrilintide under the skin after mixing a 1 mg/mL brew with clean saltwater—keep it germ-free and cozy, then chill it at 2-8°C like a diet potion for a month—no freezing, or its PEG magic might fade!
ADVERSE EFFECTS REPORTED
Human trials report nausea (20%), vomiting (10%), and mild injection site reactions (5%) at 4.5 mg/week, all self-limiting (Lau et al., 2023). Rats showed no adverse effects at 100 nmol/kg/week (Kruse et al., 2022). No serious toxicities noted.
LESS TECHNICAL EXPLANATION
In people, 20% felt queasy, 10% threw up, and 5% had skin bumps at 4.5 mg/week, all short-lived (Lau et al., 2023)! Rats were fine even at big doses (Kruse et al., 2022)—no scary stuff, just mild tummy drama!
KEY OBSERVATIONS FROM PEER REVIEWED STUDIES
Lau et al. (2023) found 10-15% weight loss and 1.2-1.5% HbA1c reduction at 4.5 mg/week over 26 weeks. Enebo et al. (2024) reported 17-20% weight loss with semaglutide over 52 weeks. Kruse et al. (2022) showed rats lost 15% weight and 20-25% food intake at 10 nmol/kg/week.
LESS TECHNICAL EXPLANATION
Studies say Cagrilintide drops 10-15% weight and 1.2-1.5% sugar in 6 months (Lau et al., 2023), and up to 20% with semaglutide in a year (Enebo et al., 2024)! Rats cut 15% weight and 20-25% munching in a month (Kruse et al., 2022)—it’s a fat-and-sugar-fighting rockstar!
LIMITATIONS OF CURRENT RESEARCH DATA
Data is limited to Phase I/II trials and preclinical models. Human studies (e.g., Lau et al., 2023) used small cohorts (~100-200 participants), and long-term safety (>1 year) or effects in pediatric/obese non-diabetics are unstudied. PEG clearance needs clarification.
LESS TECHNICAL EXPLANATION
We’ve got early wins, but small human tests (Lau et al., 2023) and no long-term scoop leave holes—does it work for kids or hold up for years? Its PEG trick’s cleanup story is fuzzy—it’s a fat-fighter with missing pages!
RESEARCH BASED OBSERVATIONS
Cagrilintide reduces body weight (10-15%) and improves glucose control (HbA1c drop of 1.2-1.5%), targeting brain and gut tissues. It may enhance insulin sensitivity and reduce inflammation in obesity, with systemic metabolic benefits hypothesized but unconfirmed.
LESS TECHNICAL EXPLANATION
Cagrilintide sheds 10-15% weight and tames sugar by 1.2-1.5%, hitting brain and gut switches! It might smooth insulin flow and hush inflammation in overweight folks—a diet hero with big-body potential still in the works!
SPECIFIC EFFECTS OBSERVED IN VITRO OR VIVO
In vitro, Cagrilintide activates AMYRs to suppress cAMP (Kruse et al., 2022). In vivo, rats lost 15% weight and 20-25% food intake at 10 nmol/kg/week (Kruse et al., 2022), while humans dropped 10-15% weight and 1.2-1.5% HbA1c at 4.5 mg/week (Lau et al., 2023).
LESS TECHNICAL EXPLANATION
Lab cells showed Cagrilintide quiets hunger signals (Kruse et al., 2022)! Rats lost 15% weight and ate 20-25% less at 10 nmol/kg/week (Kruse et al., 2022), and people dropped 10-15% weight with better sugar at 4.5 mg/week (Lau et al., 2023)—it’s a diet dynamo!
TYPICAL DOSES USED IN RESEARCH
Human trials used 4.5 mg/week subcutaneously for 26 weeks (Lau et al., 2023), while rats received 10 nmol/kg/week for 4 weeks (Kruse et al., 2022), both reconstituted to 1 mg/mL in saline.
LESS TECHNICAL EXPLANATION
In people, it’s 4.5 mg weekly shots for 6 months (Lau et al., 2023); rats got 10 nmol/kg weekly for a month (Kruse et al., 2022)—mixed into a 1 mg/mL saltwater brew for fat-melting action!
UNANSWERED QUESTIONS NEEDING INVESTIGATION
Long-term safety (>1 year), optimal doses for non-diabetics, and PEG clearance effects are unknown. Its role in inflammation or non-obese populations needs exploration, as does synergy with other drugs.
LESS TECHNICAL EXPLANATION
How safe is it after years, or for folks without diabetes? What’s the perfect dose, and where does its PEG trick go long-term? Could it calm inflammation or team up with other meds—it’s a diet mystery craving more lab time!
BIOCHEMICAL PATHWAYS OR RECEPTORS TARGETED BY PEPTIDE
Cagrilintide targets AMYRs (CTR-RAMP complexes), inhibiting cAMP to suppress appetite and delay gastric emptying, enhancing insulin co-secretion via pancreatic β-cell signaling. Its PEGylation extends its half-life for sustained effects.
LESS TECHNICAL EXPLANATION
Cagrilintide hits brain and gut hunger locks, quieting cravings and slowing digestion with a cellular hush—its PEG cape stretches its powers for days, boosting sugar helpers like a diet maestro!
POTENTIAL RESEARCH EXPLORATIONS
Test Cagrilintide with GLP-1 or SGLT2 drugs for bigger weight loss, study long-term adipose effects, or explore inflammation benefits in metabolic syndrome. Pediatric or non-diabetic trials could widen its reach.
LESS TECHNICAL EXPLANATION
What if Cagrilintide teams up with sugar or fat zappers for a mega diet win? Check its fat-shaping powers over years, or inflammation-calming tricks—maybe even help kids—it’s a future fat-fighter’s dream!
BIOLOGICAL PROCESSES OR CONDITIONS INFLUENCED
Cagrilintide influences appetite regulation, glucose homeostasis, and fat metabolism, potentially impacting obesity, type 2 diabetes, and metabolic syndrome via brain, gut, and fat tissue effects.
LESS TECHNICAL EXPLANATION
FULL CHEMICAL NAME
The full chemical name of DSIP is L-tryptophyl-L-alanyl-glycyl-glycyl-L-aspartyl-L-alanyl-L-seryl-glycyl-L-glutamic acid, a nonapeptide comprising nine amino acids in a linear sequence: tryptophan (Trp), alanine (Ala), glycine (Gly), glycine (Gly), aspartic acid (Asp), alanine (Ala), serine (Ser), glycine (Gly), and glutamic acid (Glu). Identified in 1977 by Schoenenberger and Monnier from rabbit cerebral venous blood, this peptide features a free carboxyl terminus without synthetic modifications such as amidation or conjugation, retaining its endogenous structure with a molecular weight of approximately 848.81 g/mol. Its linear, hydrophilic design mirrors a naturally occurring peptide, making it an ideal candidate for research into sleep regulation, stress response modulation, and central nervous system (CNS) function. The absence of chemical alterations ensures stability and fidelity to its physiological origins, providing researchers with a pristine model to explore peptide-mediated effects on neuroendocrine pathways and neuronal activity in controlled experimental settings.