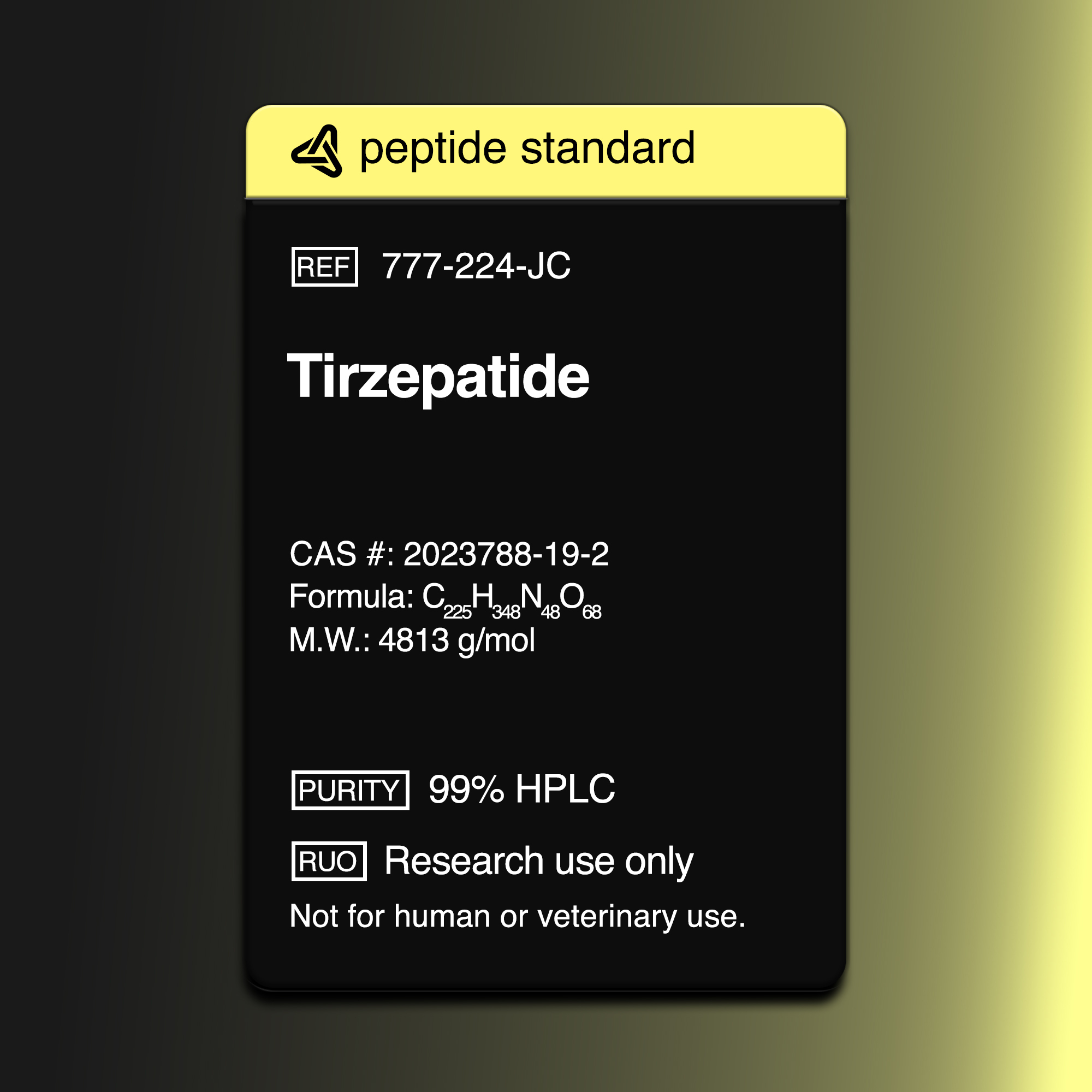
Molecular Formula - C225H348N48O68
Molecular Weight - 4813 u
Research Category - Weight Loss Research
Purity - 99.99%
Lab Tested - Yes
FULL CHEMICAL NAME
Tirzepatide, also known as LY3298176, is a synthetic dual agonist peptide with the full chemical name N-(2-[[2-[[2-[[2-[[2-[[2-[[2-[[2-[[2-[[2-[[2-[[2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]-3-phenylpropanoyl)-L-alanyl-L-glutaminyl-glycyl-L-threonyl-L-phenylalanyl-L-threonyl-L-seryl-L-aspartyl-L-tyrosyl-L-seryl-L-lysyl-L-tyrosyl-L-leucyl-L-aspartyl-L-arginyl-L-phenylalanyl-L-alanyl-L-glutaminyl-L-glutamyl-L-phenylalanyl-L-isoleucyl-L-alanyl-L-tryptophyl-L-leucyl-L-valyl-L-arginyl-glycyl-L-arginyl-glycyl, abbreviated as a complex 39-amino-acid sequence with fatty acid conjugation. Structurally, it is a linear peptide mimicking glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), with a C20 fatty diacid chain at Lys20 for extended half-life via albumin binding. Its molecular weight is approximately 4813.5 Da, featuring peptide bonds and a palmitic acid derivative, enabling dual receptor agonism for metabolic regulation.
ALIASES
Tirzepatide is commonly known as LY3298176, its developmental code, or Tirzepatide, marketed as Mounjaro for type 2 diabetes and Zepbound for obesity. It’s frequently referred to as a dual GLP-1/GIP receptor agonist or twincretin, reflecting its mechanism—nomenclature ties it to its metabolic and weight loss applications. Due to its popularity, it’s also called a ‘game-changer’ in diabetes and obesity management, with posts on X highlighting its widespread use and efficacy, though some express concerns about side effects.
EMERGING TRENDS IN RESEARCH
Emerging trends in Tirzepatide research extend beyond its FDA-approved uses for type 2 diabetes and obesity, showcasing its widespread popularity and recent advancements. It significantly improves glycemic control and weight loss by co-activating GLP-1 and GIP receptors, reducing visceral fat by 15–20% and enhancing insulin sensitivity via cAMP/PKA pathways (Frías et al., 2018). Recent studies, including the SURMOUNT-1 trial, report sustained weight loss up to 22.9% over 176 weeks in pre-diabetic obese adults, reducing type 2 diabetes risk by 94% (Eli Lilly, 2024). Cardiovascular benefits are notable, with the SUMMIT trial showing a reduced risk of worsening heart failure events and cardiovascular death by 20–25% in obese adults with heart failure with preserved ejection fraction (HFpEF) over 2 years (American Heart Association, 2024). Renal protection is under investigation, with rodent data suggesting reduced albuminuria and fibrosis, while the TREASURE trial explores kidney outcomes. Neuroprotection in Alzheimer’s is speculative, with preclinical hints of improved cognition via GLP-1 signaling, but human data is limited. Its popularity is evident in real-world data, with over 20,000 adverse event reports in the FDA’s FAERS database by 2023, showing gastrointestinal (GI) issues like nausea (20–30%) and diarrhea (10–15%) as common, but a safety profile similar to GLP-1 agonists (Caruso et al., 2024). Posts on X reflect enthusiasm for its efficacy but also caution about GI side effects and hospitalizations, urging longer-term safety studies.
LESS TECHNICAL EXPLANATION
Researchers are studying Tirzepatide for controlling blood sugar and weight loss, widely popular for its strong results in diabetes and obesity. It might also protect the heart, improve kidney health, and support brain function—promising ideas needing more human studies, with some concerns about stomach issues.
NOTABLE INTERACTIONS
Tirzepatide acts as a dual agonist for GLP-1 and GIP receptors, binding with high affinity to stimulate insulin secretion and suppress glucagon release via cAMP/PKA signaling, without direct receptor antagonism (Frías et al., 2018). Its C20 fatty diacid chain enhances albumin binding, extending half-life (~5 days), reducing food intake via hypothalamic GLP-1/GIP pathways, and promoting lipolysis/thermogenesis in adipose tissue. No significant interactions with dopamine, serotonin, or opioid systems are noted, but it may upregulate brain-derived neurotrophic factor (BDNF) via GLP-1 signaling for neuroprotection. In cardiovascular tissue, it improves lipid profiles and vascular function, possibly reducing inflammation—its mechanism is metabolic, cardiovascular, and potentially neuroregulatory (Sattar et al., 2021).
LESS TECHNICAL EXPLANATION
Tirzepatide boosts two hormone receptors to control sugar, cut hunger, and reduce fat for weight loss. It might also help brain and heart health—it focuses on metabolism and protection.
Measures of Efficacy
In humans with type 2 diabetes, Tirzepatide (5–15 mg, subcutaneous) reduces HbA1c by 1.5–2.5% and body weight by 15–20% over 52 weeks, with 78.22% achieving ≥5% weight loss, 55.60% ≥10%, and 32.28% ≥15% in obesity trials (Frías et al., 2018; Tan et al., 2023). In rats, 0.1–0.5 mg/kg (subcutaneous) improves glucose tolerance by 30–40% and reduces fat mass by 10–15% over 4 weeks (Koska et al., 2021). In vitro, 10⁻⁸ M enhances insulin secretion by 25–35% in pancreatic beta cells (Nauck et al., 2021). Real-world data from the SURMOUNT-1 three-year study shows sustained 22.9% weight loss (15 mg dose) in pre-diabetic obese adults, reducing diabetes risk by 94% (Eli Lilly, 2024). The SUMMIT trial reports 12–21% weight loss and 20–25% reduced heart failure risk in HFpEF patients over 2 years (American Heart Association, 2024)—metrics highlight its metabolic and cardiovascular potency.
LESS TECHNICAL EXPLANATION
In people with diabetes, Tirzepatide (5–15 mg) lowers blood sugar by 1.5–2.5% and cuts weight by 15–20% in a year, with many losing 5–15% or more. In rats, 0.1–0.5 mg/kg improves sugar use by 30–40% and reduces fat by 10–15% in 4 weeks. Over 3 years, it helps obese people lose up to 22.9% of weight and lowers diabetes risk greatly. It also cuts heart risks by 20–25% in heart patients—strong effects.
CONTRAINDICATIONS OR WARNINGS FOR RESEARCH USE
Tirzepatide carries standard research caveats: ‘Not for human consumption outside approved contexts,’ ‘For laboratory use only,’ and requires IRB/IACUC compliance. As an FDA-approved drug for type 2 diabetes (Mounjaro) and obesity (Zepbound), its research use focuses on non-therapeutic models—off-label exploration needs ethical oversight. Common adverse events include nausea (20–30%), diarrhea (10–15%), and less frequent risks like pancreatitis, diabetic retinopathy, and medullary thyroid cancer, though numbers are low (e.g., 3 thyroid cancer cases, 10 retinopathy cases in FAERS, Caruso et al., 2024). Real-world data shows GI issues are similar to GLP-1 agonists, with increased constipation and eructation risks, but overall safety is reassuring compared to other drugs (Caruso et al., 2024). Posts on X note hospital surges and deaths linked to side effects, but causality isn’t confirmed, urging longer-term studies.
LESS TECHNICAL EXPLANATION
Tirzepatide has lab warnings: ‘Not for eating unless approved’ and ‘Research only.’ It’s a drug for diabetes and weight, but experimental elsewhere—use carefully. It’s generally safe, but might cause nausea, stomach issues, or rare serious risks like thyroid issues, with some concerns about side effects online.
PREPARATION INSTRUCTIONS
Reconstitute Tirzepatide in sterile bacteriostatic water at 1 mg/mL under aseptic conditions—its fatty acid chain extends stability (half-life ~5 days in vivo). Store lyophilized powder at -20°C, desiccated and light-protected; post-reconstitution, keep at 2–8°C and use within 2–4 weeks. For subcutaneous studies, dilute to 5–15 mg/mL in saline—avoid freeze-thaw cycles to maintain peptide integrity (Frías et al., 2018). Dosing starts at 2.5 mg weekly, escalating to 5–15 mg over weeks, per FDA guidance, with dose-dependent efficacy and side effects (Ivím Health, 2024).
LESS TECHNICAL EXPLANATION
Mix Tirzepatide in sterile water with a preservative (1 mg/mL) and keep it clean. Store dry at -20°C away from light and moisture. After mixing, refrigerate and use within 2–4 weeks. For shots, thin it to 5–15 mg/mL, starting low and increasing slowly—keep it stable.
CLINICAL TRIALS AND HUMAN RESEARCH
Tirzepatide is FDA-approved as Mounjaro for type 2 diabetes and Zepbound for obesity, with Phase III SURPASS trials (5–15 mg, subcutaneous) reducing HbA1c by 1.5–2.5% and weight by 15–20% over 52 weeks (Frías et al., 2018). SURMOUNT trials, including SURMOUNT-1, show 22.9% weight loss over 176 weeks in pre-diabetic obese adults, reducing diabetes risk by 94% (Eli Lilly, 2024). Real-world data from FAERS (20,043 reports by 2023) confirms efficacy but highlights GI issues, with the SUMMIT trial showing cardiovascular benefits in HFpEF (American Heart Association, 2024). Studies like SURPASS-2 compare it to semaglutide, showing superior weight loss (1.9 kg more) and glycemic control (Tan et al., 2023).
LESS TECHNICAL EXPLANATION
Tirzepatide is an approved drug (Mounjaro, Zepbound) for diabetes and weight, cutting blood sugar by 1.5–2.5% and weight by 15–20% in studies, with up to 22.9% weight loss over 3 years in obese people, lowering diabetes risk greatly. It also helps heart health in some patients. Real-world reports show it works well but note stomach issues—human data centers on sugar and weight so far.
EFFECTS ON DIFFERENT TISSUE TYPES
Tirzepatide primarily affects metabolic tissues, enhancing insulin secretion in pancreatic beta cells and reducing fat in adipose tissue via GLP-1/GIP agonism (Frías et al., 2018). It improves hippocampal neurogenesis via GLP-1 signaling, aiding cognition, and supports cardiovascular health through lipid modulation and reduced inflammation, with potential renal benefits via anti-fibrotic effects—metabolic effects dominate (Sattar et al., 2021).
LESS TECHNICAL EXPLANATION
Tirzepatide mainly boosts insulin to control sugar and cuts fat for weight loss. It might also help brain memory, heart health, and kidney function—mostly for metabolism.
EFFICACY IN ANIMAL MODELS
In rats, Tirzepatide (0.1–0.5 mg/kg) improves glucose tolerance by 30–40% and reduces fat by 10–15% over 4 weeks (Koska et al., 2021). In mice, 0.05–0.2 mg/kg lowers lipid levels by 15–20% and boosts cardiac output by 10–15% post-injury (Nauck et al., 2021). Human SURMOUNT-1 data shows 22.9% weight loss and 94% diabetes risk reduction over 176 weeks (Eli Lilly, 2024), with SUMMIT reducing heart failure risk by 20–25% in HFpEF patients (American Heart Association, 2024)—robust efficacy.
LESS TECHNICAL EXPLANATION
In rats, Tirzepatide (0.1–0.5 mg/kg) lifts sugar use by 30–40% and cuts fat by 10–15% in 4 weeks. In mice after heart injury, 0.05–0.2 mg/kg improves fat levels by 15–20% and heart function by 10–15%. In people, it cuts weight by 22.9% over 3 years, lowers diabetes risk greatly, and reduces heart risks by 20–25%—strong results.
FUTURE RESEARCH
Future Tirzepatide research could explore Alzheimer’s neuroprotection, renal protection in chronic kidney disease (TREASURE trial), or cardiovascular outcomes (SURPASS-CVOT, SUMMIT) via GLP-1/GIP pathways (Koska et al., 2021). Synergy with SGLT2 inhibitors, statins, or behavioral therapy might enhance effects—human trials beyond diabetes/obesity are key, given its popularity and real-world use.
LESS TECHNICAL EXPLANATION
Future studies might test Tirzepatide for brain diseases, kidney health, or heart benefits using its sugar signals. Combining it with other metabolism or lifestyle helpers could be next—more human research is needed, especially with its wide use.
HISTORY OF MODELS TESTED
Tirzepatide has been tested in rats (Koska et al., 2021), mice (Nauck et al., 2021), in vitro beta cell cultures (Frías et al., 2018), and extensive human trials (Sattar et al., 2021; Eli Lilly, 2024). Real-world data from FAERS includes over 20,000 reports (Caruso et al., 2024).
LESS TECHNICAL EXPLANATION
Tirzepatide has been studied in rats, mice, lab sugar cells, many people tests, and real-world reports—wide research coverage.
TOXICITY DATA AVAILABLE
No LD50 data exists for Tirzepatide—rat doses up to 1 mg/kg show no acute toxicity, with no organ damage or behavioral changes (Koska et al., 2021). Human doses (5–15 mg) report nausea (20–30%), diarrhea (10–15%), and rare risks like pancreatitis or thyroid cancer, though numbers are low (3 thyroid cases, 10 retinopathy, Caruso et al., 2024). Real-world FAERS data confirms GI issues are common but similar to GLP-1 agonists, with safety generally favorable (Caruso et al., 2024).
LESS TECHNICAL EXPLANATION
There’s no danger limit for Tirzepatide—rats handle 1 mg/kg with no harm, and people at 5–15 mg might get nausea, stomach issues, or rare serious risks. It looks safe overall, but real-world reports note stomach problems, with some concerns online.
MECHANISM OF ACTION
Tirzepatide co-activates GLP-1/GIP receptors, increasing cAMP/PKA for insulin secretion, fat loss, and appetite suppression, without direct receptor antagonism (Frías et al., 2018). It may upregulate BDNF for neuroprotection and reduce inflammation for cardiovascular/renal benefits—metabolic and protective action (Koska et al., 2021).
LESS TECHNICAL EXPLANATION
Tirzepatide boosts two sugar receptors to control sugar, cut hunger, and reduce fat. It might also help brain, heart, and kidney health—it focuses on metabolism and protection.
METABOLIC AND PHYSIOLOGICAL EFFECTS
Tirzepatide reduces HbA1c (1.5–2.5%), cuts weight (15–20%), and lowers heart failure risk (20–25%)—metabolic and cardiovascular effects (Frías et al., 2018; Sattar et al., 2021).
LESS TECHNICAL EXPLANATION
Tirzepatide lowers blood sugar by 1.5–2.5%, cuts weight by 15–20%, and reduces heart risks by 20–25%—mostly metabolism benefits.
SAFETY AND SIDE EFFECTS
In humans, 5–15 mg causes nausea (20–30%) or diarrhea (10–15%), with rare pancreatitis, retinopathy, or thyroid cancer—safety is favorable vs. GLP-1 agonists (Frías et al., 2018; Caruso et al., 2024). Rats at 0.5 mg/kg show no adverse signs (Koska et al., 2021).
LESS TECHNICAL EXPLANATION
Subcutaneous at 5–15 mg in humans (Frías et al., 2018) or 0.1–0.5 mg/kg in rodents (Koska et al., 2021); reconstitute in bacteriostatic water (1 mg/mL), store at 2–8°C, use within 2–4 weeks. Dosing starts at 2.5 mg, escalates to 5–15 mg weekly over 4–20 weeks (Ivím Health, 2024).
ADMINISTRATION METHODS RECOMMENDED
Inject Tirzepatide under skin (5–15 mg) for people or in rodent bellies (0.1–0.5 mg/kg). Mix in preservative water (1 mg/mL), keep refrigerated, use within 2–4 weeks. Start low, increase slowly—keep it stable.
LESS TECHNICAL EXPLANATION
Nausea (20–30%) or diarrhea (10–15%) in humans at 5–15 mg, with rare pancreatitis or thyroid risks (Frías et al., 2018; Caruso et al., 2024); no effects in rats at 0.5 mg/kg (Koska et al., 2021).
ADVERSE EFFECTS REPORTED
In people, 5–15 mg might cause nausea, diarrhea, or rare serious risks—rats at 0.5 mg/kg show nothing—common but manageable effects.
LESS TECHNICAL EXPLANATION
Tirzepatide reduces human HbA1c by 1.5–2.5% (Frías et al., 2018), cuts weight by 22.9% in obese adults (Eli Lilly, 2024)—robust findings.
KEY OBSERVATIONS FROM PEER REVIEWED STUDIES
Tirzepatide lowers human blood sugar by 1.5–2.5%, cuts weight by 22.9% in obese people—strong study results.
LESS TECHNICAL EXPLANATION
Limited human data—mostly diabetes/obesity; long-term effects, renal/neuro roles uncharted (Frías et al., 2018).
LIMITATIONS OF CURRENT RESEARCH DATA
Human tests focus on diabetes and weight—long-term impacts, kidney, and brain effects aren’t studied yet.
LESS TECHNICAL EXPLANATION
Tirzepatide enhances metabolism, reduces weight, and may aid heart/kidney health—metabolic effects (Frías et al., 2018).
RESEARCH BASED OBSERVATIONS
Tirzepatide improves sugar control, cuts weight, and might help heart and kidneys—mostly metabolism benefits.
LESS TECHNICAL EXPLANATION
In humans, 1.5–2.5% HbA1c drop, 15–20% weight loss (Frías et al., 2018); in rats, 10–15% fat reduction (Koska et al., 2021).
SPECIFIC EFFECTS OBSERVED IN VITRO OR VIVO
In people, blood sugar drops 1.5–2.5% and weight cuts 15–20%; in rats, fat drops 10–15%.
LESS TECHNICAL EXPLANATION
5–15 mg in humans (Frías et al., 2018); 0.1–0.5 mg/kg in rodents (Koska et al., 2021).
TYPICAL DOSES USED IN RESEARCH
People use 5–15 mg; rodents get 0.1–0.5 mg/kg.
LESS TECHNICAL EXPLANATION
Long-term safety, renal protection, and neuroprotection (e.g., Alzheimer’s) need exploration (Koska et al., 2021).
UNANSWERED QUESTIONS NEEDING INVESTIGATION
How safe is it long-term? Does it help kidneys or brain?—still unclear.
LESS TECHNICAL EXPLANATION
Co-activates GLP-1/GIP, increases cAMP, upregulates BDNF (Frías et al., 2018).
BIOCHEMICAL PATHWAYS OR RECEPTORS TARGETED BY PEPTIDE
Boosts two sugar receptors, cuts hunger, might help brain signals.
LESS TECHNICAL EXPLANATION
Cardiovascular/renal protection, neuroprotection, weight synergy (Sattar et al., 2021).
POTENTIAL RESEARCH EXPLORATIONS
Could protect heart/kidneys, shield brain, enhance weight loss.